







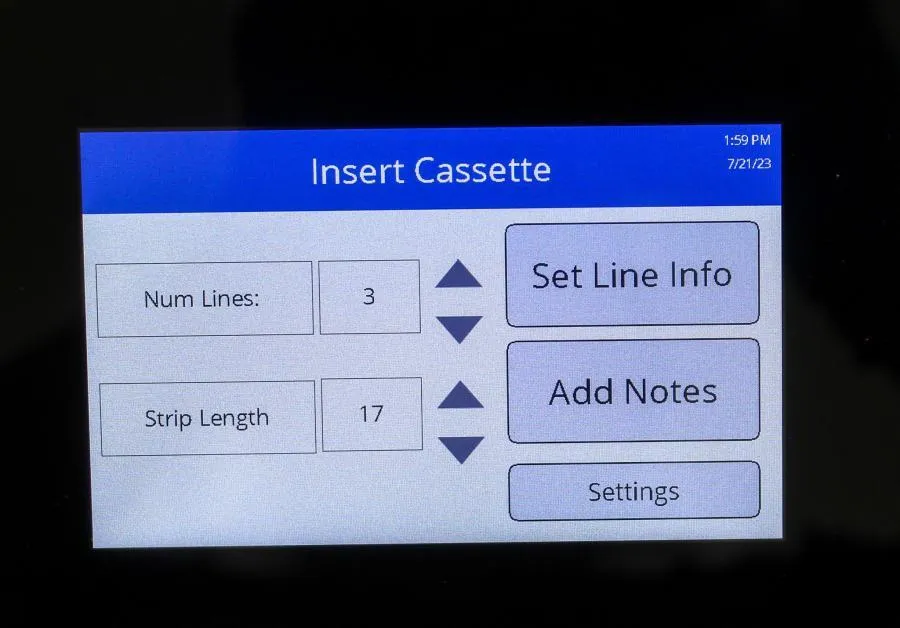



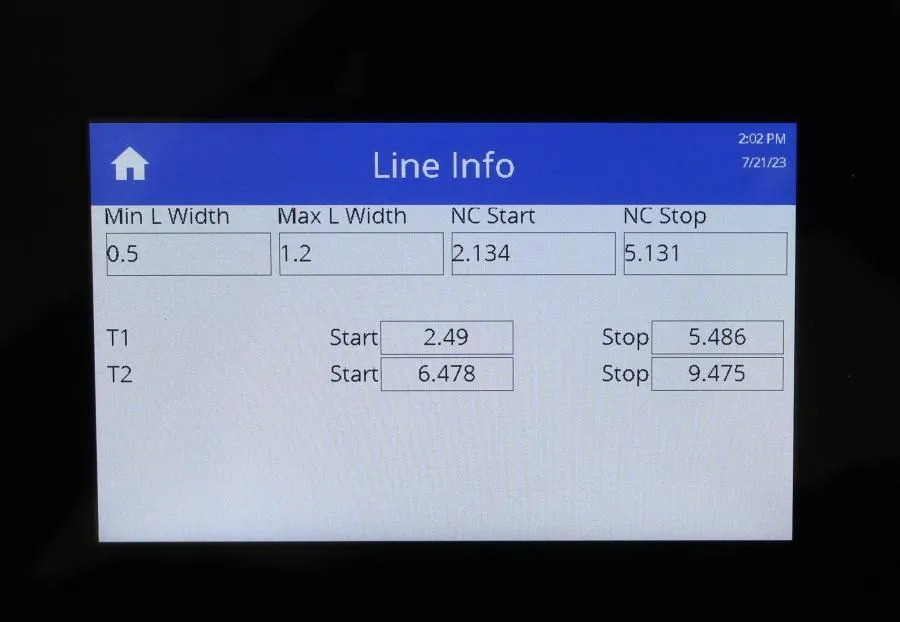

Mesa Biotech Accula AR Analizer Lot: RD2101A064
- Make MesaBiotech
- Hertz 50Hz/60Hz
- Model DARI/ Lot: RD2101A064
- Serial VV-04X
- Weight 8
- Voltage 120V
- Working Working
- Includes Power Cord
- Warranty 30-Day Warranty, 100% Parts and Labor
- Dimensions 14×14×13 in
- Shipping Type FedEx Ground
- Item Condition Pre-owned
Mesa Biotech Accula AR Analyzer Lot: RD2101A064
**Unit has been tested to specifications. Cassettes NOT included. Buyer can expected same unit as the picture with 30-Day warranty. See photos for further details.
Mesa Biotech Accula AR Analyzer is an affordable machine with sample-to-answer, CLIA-waived PCR (polymerase chain reaction) testing platform designed specifically for point-of-care (POC) infectious disease diagnosis, launched its respiratory syncytial virus test (RSV) at AACC. Mesa designs, develops, manufactures and commercializes next-generation molecular diagnostic tests, bringing the superior diagnostic performance of nucleic acid PCR amplification to the POC. Mesa’s Accula System is a sample-to-answer palm-sized, reusable dock with single-use test cassettes (NOT Included). The novel molecular test system is a visually interpreted, FDA-cleared, CLIA-waived PCR platform with the simplicity, convenience and procedural familiarity of traditional POC rapid immunoassay tests, while providing the superior sensitivity, specificity and information content of laboratory-based PCR testing accula System's RSV and Flu A/Flu B molecular tests.
The Accula Test performed on the Accula Dock or the Silaris Dock is a molecular in vitro diagnostic test utilizing polymerase chain reaction (PCR) and lateral flow technologies for the qualitative, visual detection of nucleic acid from SARS-CoV-2 in
clinician-collected nasal or nasal mid-turbinate swab specimens or clinician instructed self-collected (collected on site) nasal swab specimens, collected from individuals suspected of COVID-19 by their healthcare provider.
Testimonials
“REUZEit has been a great partner for our used equipment needs and always provide timely updates of new arrivals of consigned equipment.”
“Great company to work with. Tammy completed our first international equipment shipment with ease.”
“Fast response, open to adjusting schedule as needed, and great customer interaction.”











